Effect of Varenicline Combined With Medical Management on Alcohol Use Disorder With Comorbid Cigarette Smoking: A Randomized Clinical Trial
Cited: O'Malley SS, Zweben A, Fucito LM, Wu R, Piepmeier ME, Ockert DM, Bold KW, Petrakis I, Muvvala S, Jatlow P, Gueorguieva R. Effect of Varenicline Combined With Medical Management on Alcohol Use Disorder With Comorbid Cigarette Smoking: A Randomized Clinical Trial. JAMA Psychiatry. 2018 Feb 1;75(2):129-138. doi: 10.1001/jamapsychiatry.2017.3544. PMID: 29261824; PMCID: PMC5838706.
Affiliations 1Department of Psychiatry, Yale School of Medicine, New Haven, Connecticut. 2School of Social Work, Columbia University, New York, New York. 3Parallax Center, New York, New York. 4Department of Laboratory Medicine, Yale School of Medicine, New Haven, Connecticut. 5Department of Biostatistics, Yale School of Public Health, New Haven, Connecticut
Abstract
Importance: Individuals with alcohol use disorder have high rates of cigarette smoking. Varenicline tartrate, an approved treatment for smoking cessation, may reduce both drinking and smoking.
Objectives: To test the efficacy of varenicline with medical management for patients with alcohol use disorder and comorbid smoking seeking alcohol treatment, and to evaluate the secondary effects on smoking abstinence.
Design, setting, and participants: This phase 2, randomized, double-blind, parallel group, placebo-controlled trial was conducted at 2 outpatient clinics from September 19, 2012, to August 31, 2015. Eligible participants met alcohol-dependence criteria and reported heavy drinking (≥5 drinks for men and ≥4 drinks for women) 2 or more times per week and smoking 2 or more times per week; 131 participants were randomized to either varenicline or placebo stratified by sex and site. All analyses were of the intention-to-treat type. Data analysis was conducted from February 5, 2016, to September 29, 2017.
Interventions: Varenicline tartrate, 1 mg twice daily, and matching placebo pills for 16 weeks. Medical management emphasized medication adherence for 4 weeks followed by support for changing drinking.
Main outcomes and measures: Percentage of heavy drinking days (PHDD) weeks 9 to 16, no heavy drinking days (NHDD) weeks 9 to 16, and prolonged smoking abstinence weeks 13 to 16.
Results: Of 131 participants, 39 (29.8%) were women and 92 (70.2%) were men, the mean (SD) age was 42.7 (11.7) years, and the race/ethnicity self-identified by most respondents was black (69 [52.7%]). Sixty-four participants were randomized to receive varenicline, and 67 to receive placebo. Mean change in PHDD between varenicline and placebo across sex and site was not significantly different. However, a significant treatment by sex by time interaction for PHDD (F1,106 = 4.66; P = .03) revealed that varenicline compared with placebo resulted in a larger decrease in log-transformed PHDD in men (least square [LS] mean difference in change from baseline, 0.54; 95% CI, -0.09 to 1.18; P = .09; Cohen d = 0.45) but a smaller decrease in women (LS mean difference, -0.69; 95% CI, -1.63 to 0.25; P = .15; Cohen d = -0.53). Thirteen of 45 men (29%) had NHDD taking varenicline compared with 3 of 47 men (6%) taking placebo (Cohen h = 0.64; 95% CI, 0.22-1.03), whereas 1 of 19 women (5%) had NHDD compared with 5 of 20 women (25%) taking placebo (Cohen h = -0.60; 95% CI, -1.21 to 0.04). Taking varenicline, 8 of 64 participants (13%) achieved prolonged smoking abstinence; no one (0 of 67) quit smoking taking placebo (P = .003; Cohen h = 0.72; 95% CI, 0.38-1.07).
Conclusions and relevance: Varenicline with medical management resulted in decreased heavy drinking among men and increased smoking abstinence in the overall sample. Varenicline could be considered to promote improvements in men with these dual behavioral health risks.
Trial registration: clinicaltrials.gov Identifier: NCT01553136.
Conflict of interest statement
Conflict of Interest Disclosures: Dr O’Malley reported having been a consultant or an advisory board member for Alkermes, Amygdala, Arkeo, Cerecor, Mitsubishi Tanabe, Opiant, Pfizer; a member of the American Society of Clinical Psychopharmacology Alcohol Clinical Trials Initiative supported by Abbott, Amygdala, Ethypharm, Lilly, Lundbeck, Otsuka, Pfizer, Arbor Pharmaceuticals, and Indivior; a coinvestigator on studies receiving donated medications from Astra Zeneca, Novartis; a site principal investigator for a multisite trial by Lilly; and a scientific panel member for Hazelden Foundation. Dr Petrakis reported being a consultant to Alkermes. Dr Fucito reported registering with the US Patent and Trademark Office the name and content of a web-based program to help with sleeping and drinking (ie, Call it a Night). No other disclosures were reported.
Figures
Figure 1. Enrollment and Follow-up Flow Diagram
AUD indicates alcohol use disorder.
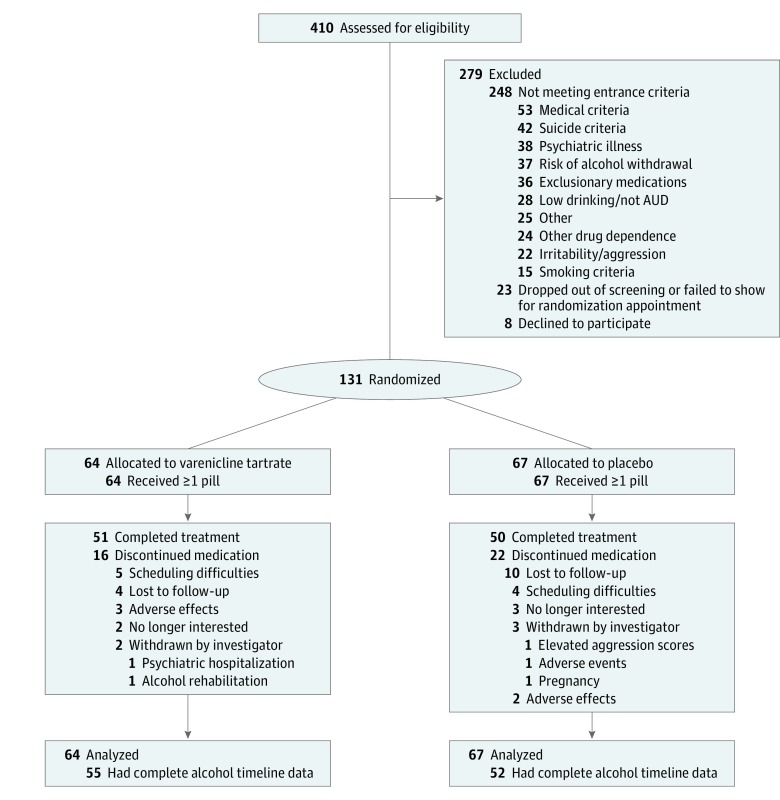
Figure 2.
Change From Baseline to End Point in Log-Transformed Percentage of Heavy Drinking Days (PHDD) Outcome by Treatment Group and Sex
Heavy drinking days were defined as 5 or more drinks within a day for men and 4 or more drinks within a day for women. Baseline was defined as the 8 weeks prior to intake. End point was defined as the last 8 weeks of the treatment period. The horizontal line in the middle of each box indicates the median, while the top and bottom borders of the box mark the 75th and 25th percentiles, respectively. The diamonds indicate mean values. The whiskers above and below the boxes mark the minimum value and the maximum value, respectively. Varenicline was given as varenicline tartrate.
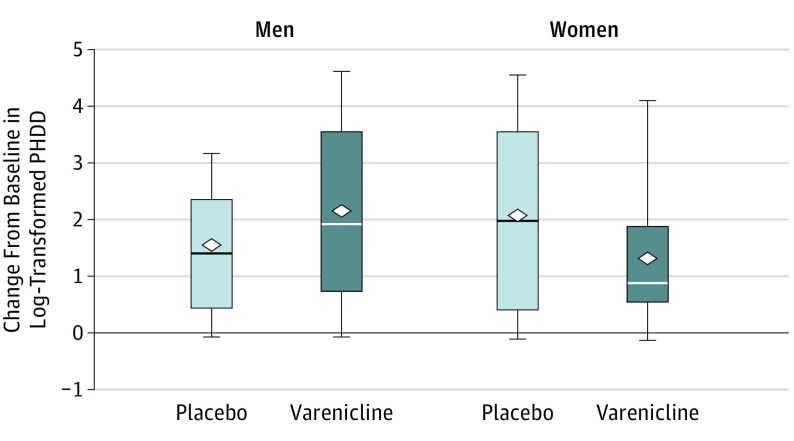
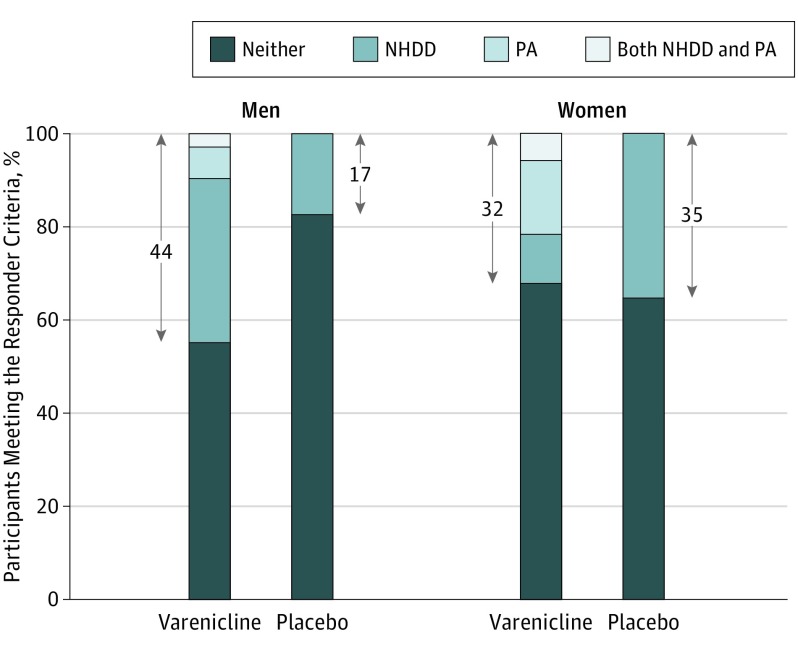
Figure 3.
Percentage of Participants Meeting the Responder Criteria by Treatment Group and Sex
Positive response on the integrated response measure was defined as either No Heavy Drinking Days (NHDD), Prolonged Smoking Abstinence (PA), or both during the last 28 days of treatment. Percentages within the arrows correspond to the percentage who had a good response on the integrated measure. Missing data were treated as nonresponse. Varenicline treatment had a higher integrated response rate than placebo for men (Cohen h = 0.60) but not for women (Cohen h = –0.06). Varenicline was given as varenicline tartrate.